Product standards
The importance of standards
The TGA, and other medical regulators worldwide set rigorous standards for the therapeutic products and medical devices that doctors and patients rely upon for peace of mind and quality assurance.
These standards ensure a minimum level of conformance for matters including:
- Product safety and design;
- Certified ingredients / prohibited ingredients;
- Accredited manufacturing facilities and cleanroom environments;
- Appropriate packaging and labelling requirements; and
- Agreed emissions standards.
In Australia, the TGA’s regulatory approach has created a pathway for therapeutic vaping products to reach licensed pharmaceutical standards over time. The most recent revision to the regulations for therapeutic vapes has brought these products closer to pharmaceutical and medical device standards.
These changes have seen suppliers leave the market, while others have been scrambling to get their products up to standard. Of course, at Liber, it’s been business as usual, as our Nicovape® Q products have always met these standards.
Liber's product standards
Device manufacturing and filling

Laboratory cleanroom standards

Toxicological risk assessment

Battery safety standards
Hazardous substances

Electromagnetic compatibility
Nicotine liquid standards
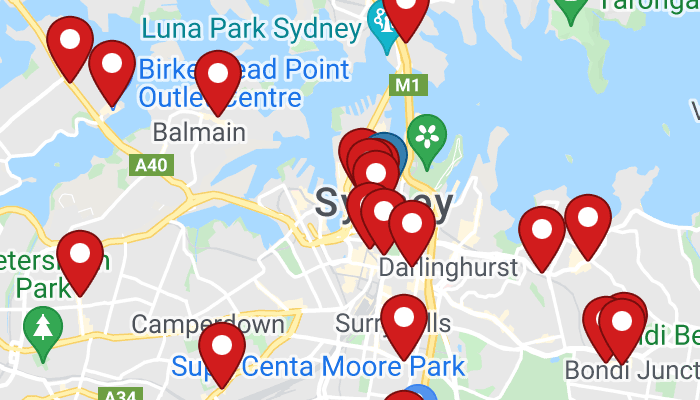
Find your nearest stockist
Over 1,000 community pharmacies across Australia currently hold nicotine vaping products in-store.
